Important forms and resources for your practice
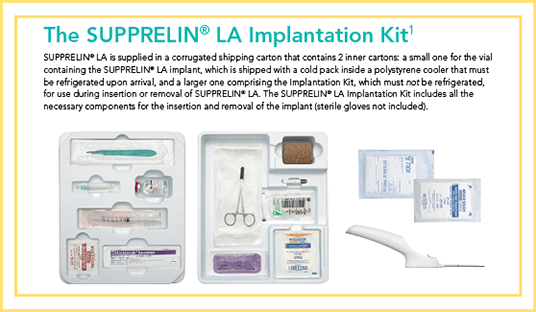
Surgical Procedure Overview
Download full instructions for implanting and removing the SUPPRELIN® LA implant
Service Request Enrollment (SRE) Form
Investigate patient’s coverage to begin the reimbursement process

Implant Removal Tip Sheet
Download this helpful tip sheet to help support SUPPRELIN® LA implant removal
Caregiver resources
All trademarks are the property of their respective owners.