A full year of consistent medication delivered in a single, small, flexible implant1
Consistently delivers ~65 mcg of histrelin acetate every day for 1 full year.1
Minimally invasive procedures1:
- Insertion and removal are minimally invasive surgical procedures that may be performed under local anesthetic using aseptic technique
- The method of anesthesia used (ie, local, conscious sedation, general) is at the discretion of the healthcare professional
- For more information on the suggested insertion and removal procedure, please see the full Prescribing Information
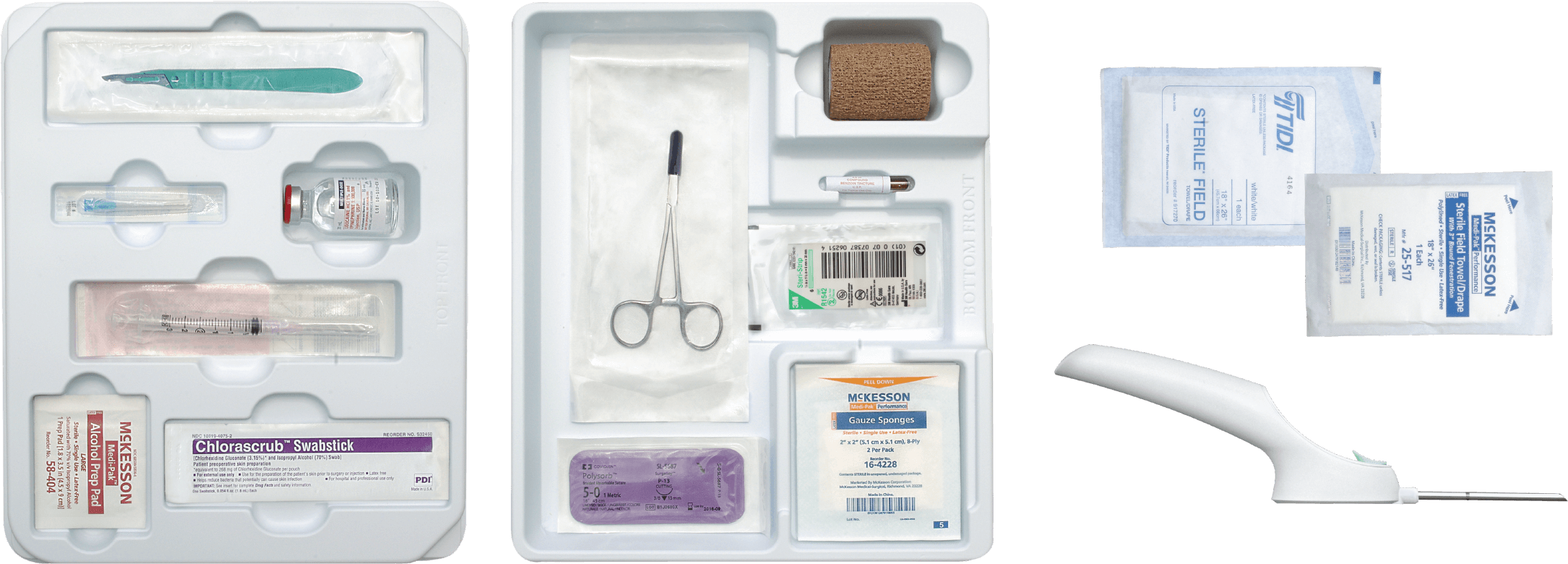
The SUPPRELIN® LA Implantation Kit1
SUPPRELIN® LA is supplied in a corrugated shipping carton that contains 2 inner cartons: a small one for the vial containing the SUPPRELIN® LA implant, which is shipped with a cold pack inside a polystyrene cooler that must be refrigerated upon arrival, and a larger one comprising the Implantation Kit, which must not be refrigerated, for use during insertion or removal of SUPPRELIN® LA. The SUPPRELIN® LA Implantation Kit includes all the necessary components for the insertion and removal of the implant (sterile gloves not included).
The Implantation Kit is to be stored at room temperature and should NOT be refrigerated.
Components
- 1 #15 disposable scalpel
- 1 syringe with 18-gauge needle
- 1 25-gauge, 1.5” needle
- 1 SS mosquito clamp
- 4 packages gauze sponges
- 2 packages alcohol swabs
- 1 fenestrated drape
- 1 non-fenestrated drape
- 1 package skin antiseptic swab
- 1 package surgical closure strips
- 1 package coated absorbable suture
- 1 package cohesive bandage
- 1 vial local anesthetic (manufacturer may vary)
- 1 implant insertion tool
- 1 benzoin tincture antiseptic
- Prescribing Information, including Medication Guide*
Item not shown.
The SUPPRELIN® LA implant contains 50 mg of histrelin acetate. The SUPPRELIN® LA implant carton contains a cold pack for refrigerated shipment and a small carton containing an amber plastic pouch. Inside the pouch is a glass vial with a Teflon-coated stopper and an aluminum seal, containing the implant in 2 mL of sterile 1.8% sodium chloride solution. (Note: The 3.5-mL vial is not completely filled with saline.) Upon receipt, refrigerate the small carton containing the amber plastic pouch and glass vial (with the implant inside) until the day of insertion. The implant vial should not be opened until just before the time of insertion.1
Coding is part of the clinical decision. Please use codes that most accurately reflect the procedures performed. Suggestions by Endo do not guarantee reimbursement or take the place of professional coding advice.
